Contact us
For General Inquiries Email: info@nirmidas.com
For Product Support Email: support@nirmidas.com
HIGH PERFORMANCE pGOLD™ MULTIPLEXED IGG/IGM ASSAY FOR COVID-19 SEROLOGICAL (WHOLE BLOOD/SERUM) AND SALIVA SAMPLES WITH AVIDITY AND NEUTRALIZATION ASSAYS
OTHER PGOLD™ ASSAYS INCLUDE ZIKA/DENGUE AND RELATED, TORCH RELATED, AUTOIMMUNE RELATED.
OVER 60 POINT-OF-CARE TESTS BASED ON FLUORESCENCE DETECTION AND CONVENTIONAL LATERAL FLOW DEVICES, INCLUDING MIDASPOT® COVID-19 ANTIBODY DETECTION KIT AND ITS CONTROL KIT, MIDASWIFTTM SARS-CoV-2 ANTIGEN DETECTION KIT, HEPATITIS C SCREENING TEST KIT.
See Related Publications:
Zika/Dengue: See American Journal Tropical Medicine and Hygiene, Nature Medicine
Toxo: See Diganostic Microbiology and Infectious Disease, Journal of Clinical Microbiology
COVID-19: See Nature Biomedical Engineering
Peptide Array Detection: PLOS
HOW THE COVID-19 TESTS CAN BE USED
The test can be used for a variety of samples including serum, plasma, whole blood, dry blood spot, and saliva in a lab facility. It can be used to assess IgG and IgM levels in a sample with 100% sensitivity and 99.87% specificity without interferences from other diseases. Avidity pGOLD™ assay can tell if a COVID-19 infection is recent or remote greater than 6 months ago.
DIFFERENTIATORS OF THE PGOLD™ COVID-19 IGG/IGM ASSAY KIT
pGOLD™ assay afford high sensitivity, > 87% for IgM > 5 days post symptom onset and ~ 100% for IgG and IgM 15 days post symptom onset.
pGOLD™ assay affords specificity > 99.5 and is more specific than many other tests known, with nearly ~ 0 cross-reactivity with other coronaviruses/common colds and other infectious diseases or autoimmune diseases.
pGOLD™ assay is semi-quantitative, simultaneously detecting antibodies and avidity against a panel of coronaviruses.
pGOLD™ assay is capable of detecting low concentrations of antibodies in saliva samples. Thus, the high sensitivity of SARS-CoV-2 antibody detection in human saliva samples on the novel nanotechnology based on pGOLD™ substrate has potential to enable non-invasive home sample collection for screening of COVID-19.
PRODUCT OVERVIEW
Nirmidas Biotech Inc. announces the availability of pGOLD™ COVID-19 IgG/IgM Assay Kit, a high performance, semi-quantitative assay for detecting IgG, IgM and IgG avidity against the S1 subunit of the spike protein, the receptor binding domain (RBD) of SARS-CoV-2 spike protein and other coronaviruses on the pGOLD™ substrate in human serum, plasma and saliva sample types. The pGOLD™ assay has been peer-reviewed and is published in Nature Biomedical Engineering on October 29, 2020 (https://www.nature.com/articles/s41551-020-00642-4).
The pGOLD™ COVID-19 IgG/IgM Assay is in an ELISA type of format. Requiring only 1 micro-liter of patient sera/plasma or 50 ml of saliva. Each pGOLD™ biochip can run 48 samples with controls in each run that takes about 2 hours to complete. In each assay run, one obtains the IgG and IgM status against multiple coronavirus antigens (including SARS-CoV-2, SARS and various common coronaviruses) for each of the 48 samples.
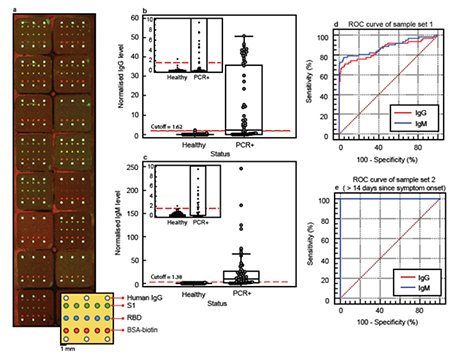
Figure. 1 | A nano-plasmonic platform for SARS-CoV-2 antibody testing. (a) An overlay of confocal fluorescence scanned images of IgG (green) and IgM (red) channels acquired after testing serum samples in isolated wells (square-shaped regions). Yellowish-green coloured spots correspond to the detection of both IgG and IgM in the sample. The lower right schematic drawing shows the printing layout of S1 (in green) and RBD (in blue) antigens and human IgG control spots (in white) in each well. The BSA-biotin spots (in red) are always labelled by a streptavidin dye to be detected in the IgM fluorescence channel and serve as an intrawell signal normaliser. (b) IgG levels against S1 detected in PCR-negative COVID-19 or presumptive negative (‘Healthy’, n = 384) and PCR-positive (‘PCR+’, n = 62) COVID-19 samples normalised by a factor of 10 and a cutoff value of 1.62 indicated as a red dashed line. The inset shows a close-up of normalised IgG levels of healthy and PCR+ samples near the cutoff with one false positive. (c) The same as (b) except for IgM levels normalised by a factor of 100 and a cutoff value of 1.38 indicated as a red dashed line. The inset shows a close-up of normalised IgM levels of healthy and PCR+ sample near the cutoff with one false positive. (d) ROC curve for pGOLD SARS-CoV-2 IgG/IgM assay based on 384 negative and 62 PCR-positive COVID-19 sera (”sample set 1”), which were used to establish IgG and IgM cutoffs. (e) ROC curve for pGOLD SARS-CoV-2 IgG/IgM assay based on 384 negative and 33 PCR-positive COVID-19 serum samples collected 15 to 45 days post symptom onset (a subset of “sample set 2”). Data in (b) and (c) are presented as box plots with the minima, maxima, median line, interquartile range, data points, and outliers shown.
KEY FEATURES
Superior Sensitivity and Specificity
The pGOLD™ COVID-19 IgG/IgM assay is superior in sensitivity, specificity and analytical sensitivity. It detects ~ 5 logs of antibody concentrations with ~ 4-5 logs of signal dynamic range, owing to nano-plasmonic enhanced NIR fluorescence detection by up to ~ 100 times (see Nature Medicine, DOI: 10.1038/nm.4302).
The pGOLD™ COVID-19 IgG/IgM assay affords > 87% sensitivity for IgM detection for COVID-19 patients > 5 days after disease symptom onset, and ~ 100 % sensitivity for both IgG and IgM > 14 days post symptom onset. It affords a 99.78% specificity based on testing 454 pre-pandemic and PCR confirmed negative samples without any cross-reactivity with other respiratory diseases and infections such as HIV, HBV, Zika, Dengue and autoimmune diseases. The pGOLD™ SARS-CoV-2 IgG/IgM Assay is promising for aiding diagnosis, population-based mass-screening, and sero-surveillance and prevalence studies. The high sensitivity in IgM detection prior to IgG onset could disease assessment in the early stage.
Multiplexed Antibody Avidity Measurement
The pGOLD™ assay is multiplexed, detecting IgM and IgG antibodies and IgG avidity against multiple antigens of SARS-CoV-2, SARS and common human coronaviruses in a single test run. Antibodies developed shortly after a primary infection exhibit low avidity and bind weakly to the antigen. Over time, avidity towards antigens can increase as antibodies ‘mature’. Antibody avidity is useful to aid differentiation of recent from past infection and distinguish primary from secondary infection. Antibody avidity could also be useful to assessing vaccination efficacy, immunity, and convalescent plasma based antibody therapy. It can also help to decipher cross-reactivity between various human coronaviruses.
Detection of SARS-CoV-2 IgG in Human Saliva
It is well known that the antibody concentration in human saliva is orders of magnitude lower than in blood or serum, demanding assay platforms with exquisite analytical sensitivity and capable of detecting ultra-high signal over background noise. The pGOLD™ assay detected IgG in saliva samples of 10 fully recovered, PCR-confirmed COVID-19 individuals and detected IgG in 8 out of 10 saliva samples from patients infected > 10 days prior to saliva collection. pGOLD™ assay also revealed that a substantial percentage of saliva from the recently infected patients also showed positive for IgM contrary to that of fully recovered patients tested previously. The results suggest the potential of antibody testing on pGOLD™ through non-invasive sample matrices such as saliva, which could greatly facilitate COVID-19 diagnosis, surveillance and prevalence studies.
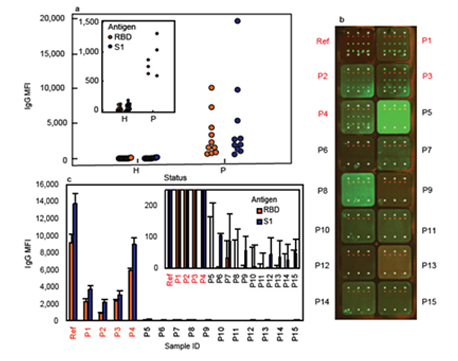
Figure. 2 | Detection of SARS-CoV-2 antibodies in human saliva.(a) Distribution of IgG median fluorescence intensity (MFI) signals from the saliva of COVID-19 positive patients (“P”, n = 10) compared to healthy controls (“H”, n = 23) against both S1 and RBD, with the inset showing a noticeable difference between the weak IgG signals from COVID-19 patient saliva and signal noise from that of healthy saliva. (b) A confocal fluorescence image of IgG signals in the saliva of recovered COVID-19 patients (n = 4, denoted as P1-P4 in red) and healthy controls (n = 11, denoted as P5-P15) and a 10⁴ times diluted serum of a PCR-confirmed COVID-19 patient as a reference (denoted as Ref in red). Here the human IgG control spots are located on row 1 and 7, intrawell signal normaliser spots are on row 2, RBD spots are on row 4, and S1 spots are on row 6 in each microarray. (c) The MFI signals of anti-S1 and anti-RBD IgG measured in saliva samples (P1-P15) and PCR-positive COVID-19 serum reference (Ref) from the biochip seen in (b) with background signals subtracted. MFI of healthy controls were near background noise as seen in the inset. Error bars indicate one standard deviation from the mean of the MFI signals detected against the respective microarrayed antigen spots (n = 3).
OTHER ASSAYS
Nirmidas has also developed other multiplexed assays on pGOLDTM assay platform
- Zika/Dengue and related
- Toxoplasmosis and ToRCH related
- Autoimmune disease autoantibody assays
60+ point-of-care diasgnostic assays available upon request. They include infectious diseases (e.g., HCV for screening), cardiovascular, and other assays for chronic disease monitoring or disgnostics. please inquire at Please inquire at info@nirmidas.com.
CUSTOMIZED ASSAY & TESTING SERVICE
Nirmidas can provide RUO service on customized assays on the pGOLD™ platform, with any coronavirus related antigens provided by Nirmidas or customers in single-plex or multiplexed formats. The biochips can be read by existing Western blot type of instrument or by Nirmidas’ MidaScan™ dual fluorescence reader. Testing service for samples provided by customers to report IgG and IgM status against multiple coronavirus antigens for each sample for research use is also provided. Please inquire at info@nirmidas.com.
This product is available for research use now!
Nirmidas pGOLD™ COVID-19 IgG/IgM Assay Kit is available for purchase today. Please fill out the inquiry form below or email us at info@nirmidas.com for price inquiry, order placement, or any product-related information.
Inquiry form
Please fill out the following form to help us understand your needs better. One of our team members will reach out to you shortly.